How brilliant minds transformed a philosophical idea into the foundation of modern physics
Have you ever wondered what happens if you keep cutting something smaller and smaller? 🤔 This simple question launched humanity’s greatest scientific adventure, transforming our understanding from solid matter to quantum clouds and revolutionizing everything from medicine to computers.
The Ancient Mystery 🏛️
Around 400 BCE, Greek philosopher Democritus proposed that everything was made of tiny, indivisible particles called “atomos” (meaning uncuttable). But this remained just philosophy for over 2000 years. Why? Because atoms are impossibly small—a single water drop contains 1.67 × 10²¹ molecules! If each molecule were marble-sized, that drop would be larger than Earth itself! 🌍
Dalton’s Solid Spheres: Chemistry Gets Scientific ⚗️

In 1803, English schoolteacher John Dalton made the atomic theory scientific. Working in Manchester with simple equipment, he discovered that elements always combine in fixed ratios. When carbon bonds with oxygen, the mass ratios are always exactly 1:8 for water formation—not approximately, but exactly! This precision suggested matter consists of discrete, unchanging units.
Dalton proposed atoms were solid, indivisible spheres—like tiny billiard balls. Each element had identical atoms with specific masses, and chemical reactions just rearranged these atoms. This transformed chemistry from art to quantitative science, laying groundwork for the periodic table and modern chemical industry.
But Dalton’s model couldn’t explain electrical phenomena or why heated elements emit specific colors. The atom’s story was just beginning.
Thomson’s Electron Discovery: The Plum Pudding Revolution 🍮
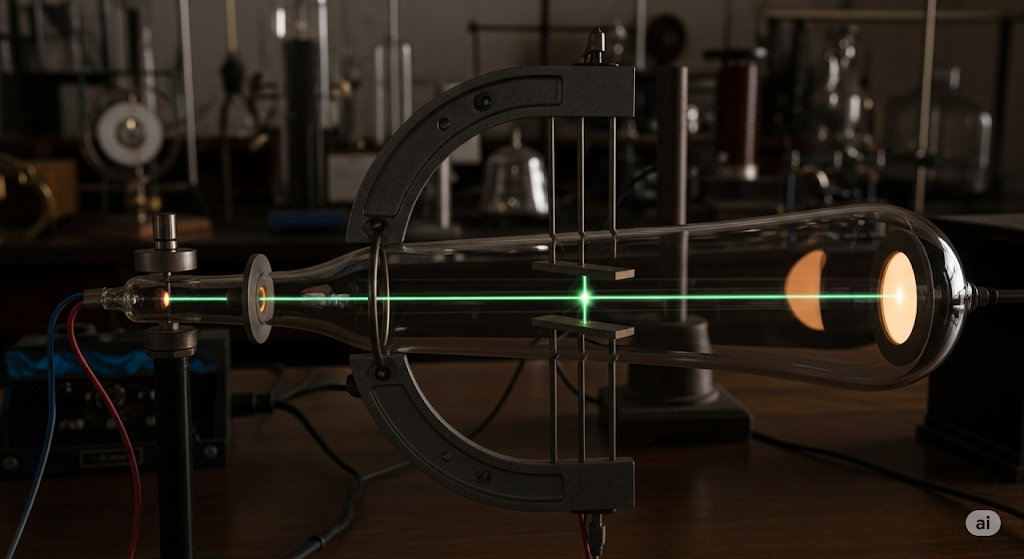
In 1897, Cambridge physicist J.J. Thomson was experimenting with cathode ray tubes (early TV screens). He discovered these rays were streams of incredibly light, negatively charged particles—electrons! His charge-to-mass calculation revealed e/m = 1.76 × 10¹¹ C/kg, nearly 2000 times larger than hydrogen ions.
This shattered Dalton’s indivisible atom. Atoms contained smaller parts! Since atoms are electrically neutral, Thomson proposed his “Plum Pudding Model“—atoms as spheres of positive charge with electrons embedded throughout, like plums in pudding.
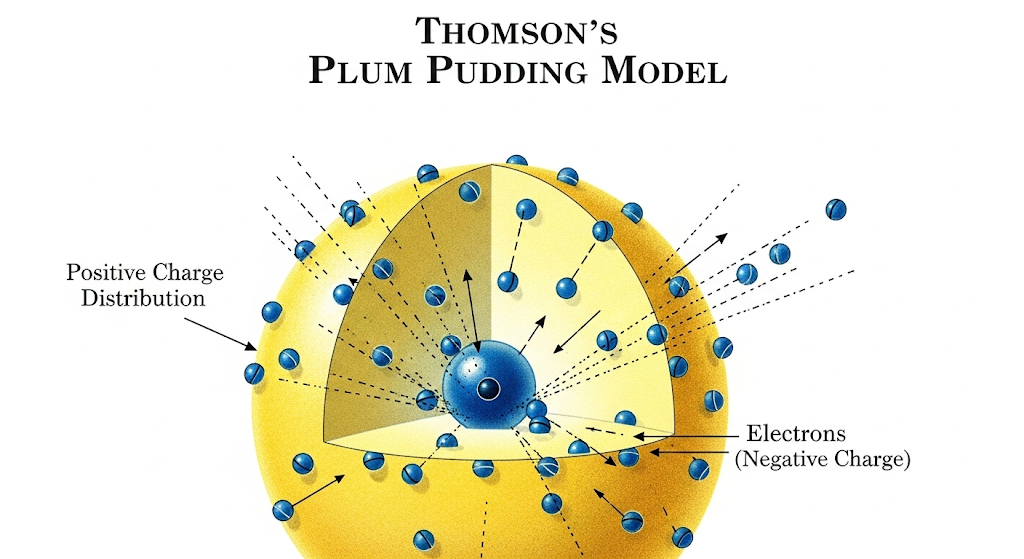
Thomson’s discovery explained electrical phenomena and introduced the first subatomic particle. Every electronic device today traces back to his cathode ray experiments. But his model faced a crucial test that would reveal its fatal flaw.
Rutherford’s Nuclear Shock: The Mostly Empty Atom 🎯💥
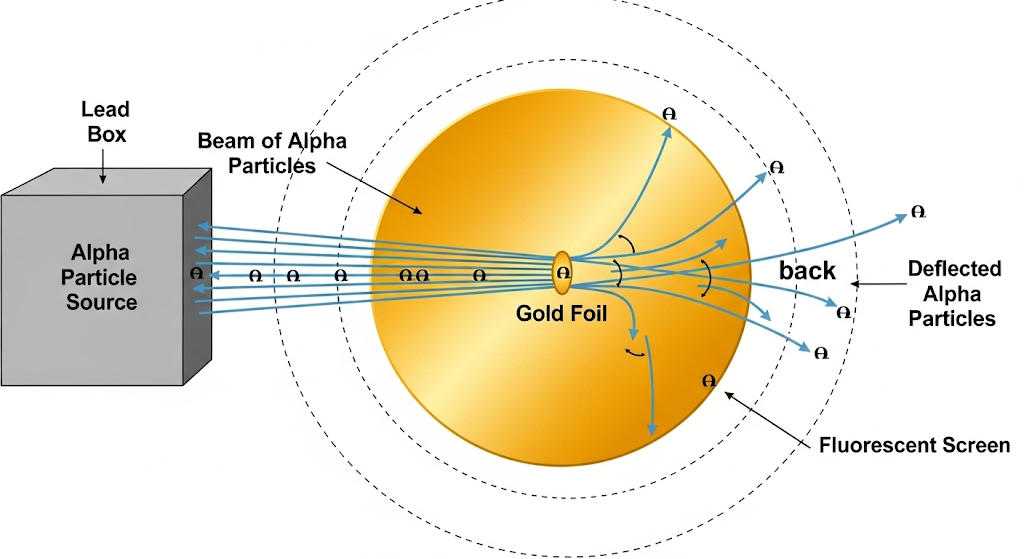
In 1911, Ernest Rutherford (Thomson’s former student) conducted an experiment that would stun the scientific world. He fired alpha particles at incredibly thin gold foil, expecting them to pass straight through Thomson’s “pudding.”
The results were shocking! While 99.95% passed through unchanged, some deflected at large angles, and 1 in 8000 bounced straight back! Rutherford famously said: “It was as incredible as firing a shell at tissue paper and having it bounce back and hit you.”
The Mathematical Revelation
Rutherford’s scattering analysis revealed that atoms contain a tiny, incredibly dense nucleus less than 10⁻¹⁴ meters across, containing virtually all the atom’s mass. To visualize this: if an atom were a football stadium, its nucleus would be smaller than a marble at center field!
This discovery revealed atoms as mostly empty space, with electrons orbiting a tiny nucleus like planets around the sun. But classical physics predicted disaster—orbiting electrons should radiate energy and spiral into the nucleus in 10⁻¹¹ seconds, making atoms impossible! Yet atoms exist, so classical physics was fundamentally wrong.
Bohr’s Quantum Breakthrough: Electron Energy Levels 🌟🔄
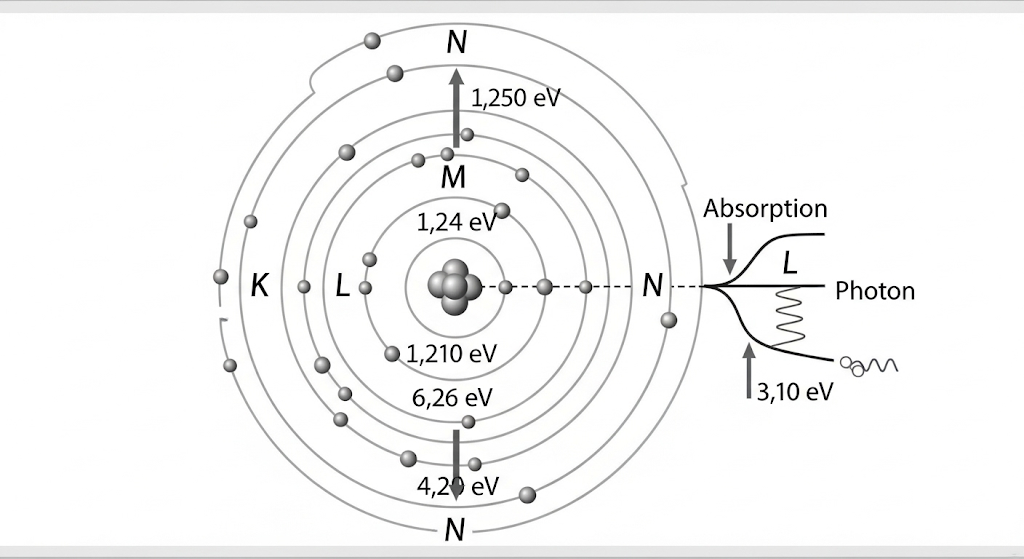
Danish physicist Niels Bohr solved this crisis in 1913 with a revolutionary proposal. He abandoned classical physics entirely for atomic systems, proposing that electrons exist only in specific, quantized energy levels around the nucleus.
Bohr’s three radical postulates violated classical physics but explained atomic stability. Electrons occupy fixed orbits where angular momentum equals nℏ (where n is an integer and ℏ is Planck’s constant). They emit or absorb light only when jumping between these levels, with photon energy exactly matching the energy difference.
The Mathematical Success
For hydrogen, Bohr calculated energy levels as E_n = -13.6 eV/n² and orbital radii as r_n = n² × 0.529 Å. These formulas precisely predicted hydrogen’s spectral lines! The famous red hydrogen line (656 nm) results from electrons dropping from level 3 to level 2, matching experimental observations perfectly.
Bohr’s model explained atomic stability, spectral lines, and chemical periodicity. However, it failed for multi-electron atoms and couldn’t explain chemical bonding. The model treated electrons as classical particles following definite orbits—an assumption that would soon be challenged.
Schrödinger’s Wave Revolution: The Quantum Reality 🌊🔬
By 1926, physics faced another revolution. Louis de Broglie proposed that particles exhibit wave properties, while Werner Heisenberg developed uncertainty principles. Austrian physicist Erwin Schrödinger created the mathematical framework still used today by treating electrons as wave phenomena.
Schrödinger’s wave equation Ĥψ = Eψ describes electrons through wavefunctions ψ containing all possible information about quantum systems. Unlike classical physics with definite particle positions, quantum mechanics deals in probabilities. The quantity |ψ|² gives the probability density of finding an electron at any location.
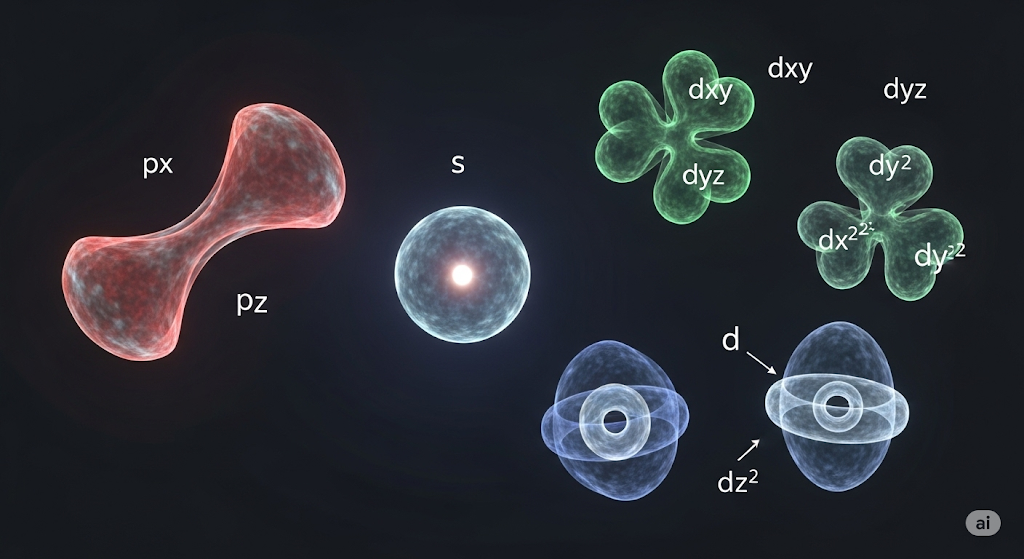
This meant abandoning classical orbits entirely. Electrons exist in “orbitals”—three-dimensional probability clouds around the nucleus with characteristic shapes determined by quantum numbers. These aren’t planetary orbits but probability distributions where electrons are likely to be found.

Quantum Numbers and Atomic Architecture
Four quantum numbers describe electron states: n determines energy and size, ℓ creates orbital shapes (s spheres, p dumbbells, d complex forms), mℓ controls orientation, and ms represents intrinsic spin. These numbers explain the periodic table structure through electron configuration patterns.
Heisenberg’s Uncertainty Principle
Quantum mechanics revealed fundamental measurement limitations: Δx × Δp ≥ ℏ/2. This isn’t experimental limitation but nature’s fundamental property. The uncertainty principle explains why electrons don’t collapse into nuclei—quantum “pressure” balances electrostatic attraction, determining atomic size and stability.
The quantum mechanical understanding of atoms drives today’s technology revolution. Semiconductors in every electronic device exploit quantum band theory—electrons occupying energy bands separated by forbidden gaps. Doping silicon with phosphorus or boron creates controllable electrical properties enabling transistors and microprocessors.
Lasers directly exploit atomic energy levels through stimulated emission. When excited atoms encounter photons matching their transition energies, they emit identical photons—same energy, phase, and direction. This creates coherent light beams used in everything from fiber optics to medical surgery.
Magnetic resonance imaging (MRI) uses nuclear spin properties. Atomic nuclei in magnetic fields have split energy levels with frequencies f = γB₀/2π. Radiofrequency pulses induce transitions analyzed by computers to create detailed body images without radiation.
Atomic clocks achieve incredible precision by using cesium-133 transitions as natural oscillators. These clocks define the international second and enable GPS navigation with accuracy better than one second over the universe’s age!
The Quantum Future 🌟
Current research pushes atomic physics into exotic territories. Quantum computers use individual atoms as qubits, exploiting superposition and entanglement for calculations impossible with classical computers. Bose-Einstein condensates merge ultra-cold atoms into single quantum entities visible to the naked eye.
Quantum sensors using individual atoms detect gravitational waves and search for dark matter. The atomic model’s evolution from philosophical speculation to quantum precision represents humanity’s greatest intellectual achievement, with each breakthrough revealing deeper reality layers while enabling civilization-transforming technologies.
The Continuing Adventure ⚛️✨
For physics enthusiasts, this journey continues. The atom, once thought indivisible, became a window into reality’s fundamental nature. From Democritus’s philosophical atoms to Schrödinger’s quantum clouds, each step required abandoning cherished assumptions and embracing nature’s deeper truths.
Today’s quantum technologies would seem magical to early atomic theorists, yet they’re direct descendants of basic research into matter’s fundamental structure. The next chapter remains unwritten, waiting for curious minds to push boundaries even further into the quantum realm.
What mysteries will the next generation uncover? The universe keeps revealing its secrets to those bold enough to ask the right questions. 🚀
Key Takeaway: The atomic model’s evolution demonstrates how human curiosity, mathematical precision, and experimental ingenuity combine to unlock nature’s deepest secrets—transforming philosophical speculation into the technological foundation of our modern world.